2025 Clinical Trials Overview: Growing Evidence Base

Clinical trials are structured research studies that test the safety and effectiveness of new therapies, devices, or medical procedures in humans. In 2025, clinical trials are more data-driven, tech-enabled, and globally coordinated than ever.
Key Characteristics of 2025 Clinical Trials:
- Phases I–IV: Evaluate safety, dosage, efficacy, and post-market surveillance.
- Trial Design: Includes randomized control, double-blind setup, or crossover methods.
- Data Oversight: Involves IRB approval, informed consent, and regular monitoring.
- Technological Tools: Use of digital biomarkers, EDC systems, and decentralized trial platforms.
- Patient Enrollment: Involves eligibility screening, diagnostic criteria, and demographic balance.
The increasing number of trials in medicine reflects both innovation in healthcare and the urgency to meet diverse therapeutic needs.
What Is Scrambler Therapy in Clinical Trials?
Scrambler Therapy is a non-invasive, neurostimulation-based pain treatment that delivers artificial nerve signals to confuse or “scramble” chronic pain messages.
How It Works:
- Mechanism: Uses a Scrambler device to transmit synthetic nerve signals via surface electrodes.
- Target: Acts on the dorsal root ganglion, disrupting pain signal transmission.
- Purpose: Alters the way pain is perceived without drugs or invasive procedures.
How It's Different from TENS:
Scrambler Therapy has shown efficacy in managing neuropathic pain, cancer pain, fibromyalgia, and post-surgical pain.
Current Status of Clinical Trials in 2025
Clinical research has expanded significantly in 2025, driven by new technologies and global cooperation.
Data Snapshot:
- ClinicalTrials.gov lists over 460,000 registered trials worldwide.
- WHO ICTRP shows active recruitment in over 140 countries.
- The EMA and NIH have increased regulatory flexibility to accelerate innovative therapies.
Top Regions Leading in Trial Volume:
- United States – biotech-heavy and diversified by trial phase
- EU Countries – strong ethical oversight and multi-country collaborations
- Asia-Pacific – large patient pools and fast-growing biotech sectors
Clinical trial advancements in 2025 emphasize real-time data, adaptive protocols, and improved transparency via open-access registries.
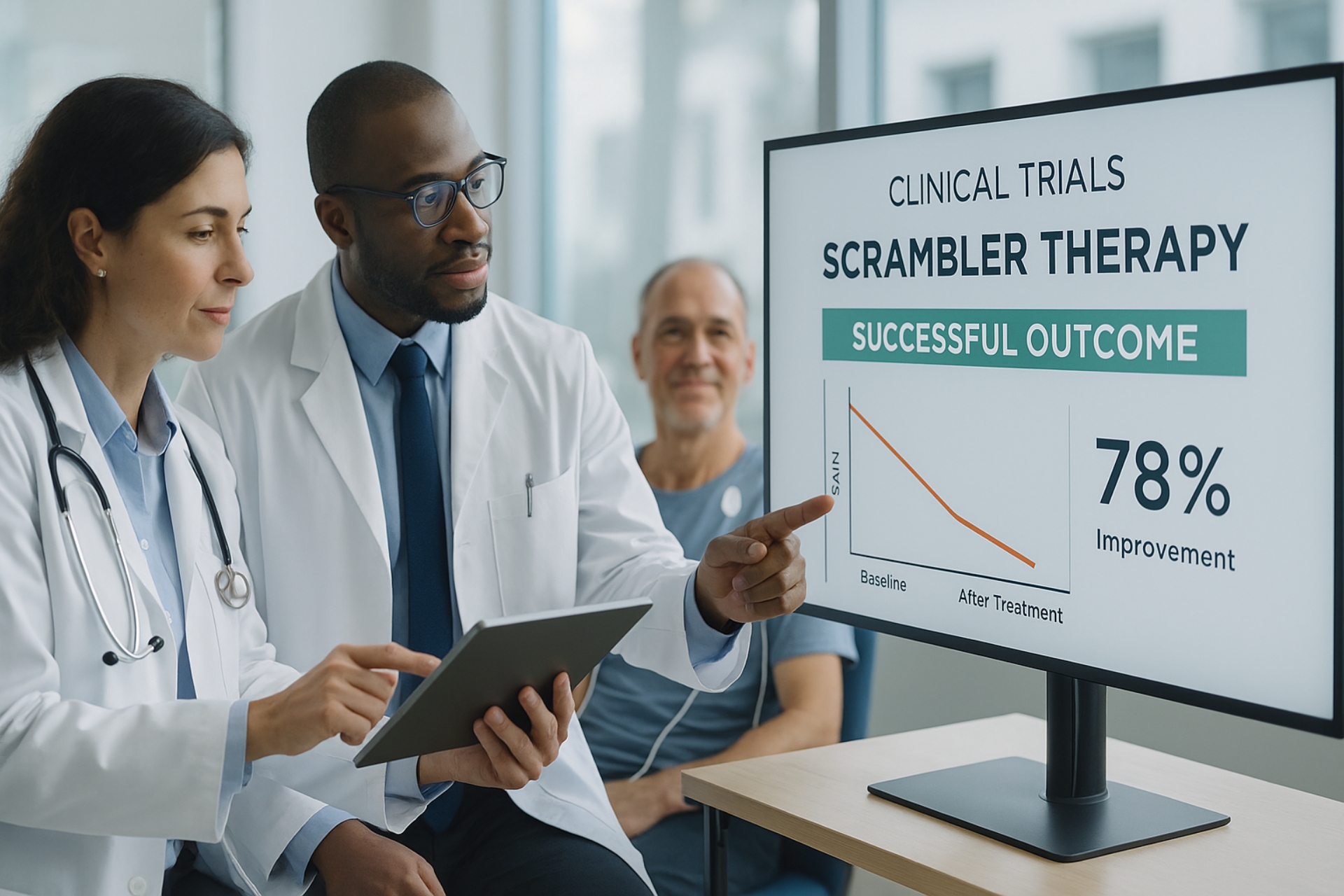
Breakthrough Scrambler Therapy Trials in 2025
Several prominent research institutions, including Mayo Clinic and National Cancer Institute (NCI), are conducting RCTs involving Scrambler Therapy.
Highlights from 2025 Trials:
- RCT in diabetic neuropathy: Showed 58% average reduction in pain over 3 weeks
- Post-chemo pain study: 41% improvement in patient-reported outcomes on the VAS scale
- Pilot study on fibromyalgia: Indicated a 62% response rate with no severe side effects
Researchers continue to monitor treatment frequency, side-effect profiles, and long-term efficacy through extended follow-ups.
Clinical Trial Phases and Methodology
Understanding trial structure helps interpret where Scrambler Therapy stands in research progression.
Clinical Trial Phases Explained:
- Phase 0: Micro-dosing, early mechanism insight
- Phase I: Safety and dosage in small groups
- Phase II: Efficacy, side effects, treatment dose
- Phase III: Comparison with current standards
- Phase IV: Post-market safety and real-world data
Scrambler Therapy-Specific Methodology:
- Randomization: Equal distribution into control and treatment arms
- Double-Blind Design: Reduces bias
- Placebo Control: Uses inactive devices for comparison
- Endpoints: Focus on analgesic effect, functional ability, and life quality
Trials use clinical protocols and data monitoring committees to ensure validity and participant safety.
Key Outcomes from Scrambler Therapy Clinical Trials
Recent trials have provided measurable, patient-centered outcomes that support the potential of Scrambler Therapy.
Reported Outcomes:
- Pain Reduction: Average 3–4 points drop on the VAS (0–10) scale
- Improved Functionality: Greater physical activity tolerance
- Patient-Reported Outcomes (PROs): Notable gains in daily quality of life
- Low Adverse Events: Less than 4% report minor skin irritation
Patients in follow-up assessments have maintained benefits up to 6 months post-treatment.
Scrambler Therapy trials continue to collect baseline pain data and track long-term relief.
Patient Enrollment and Inclusion Criteria
Eligibility to join a Scrambler Therapy study is determined by strict clinical protocols.
Common Inclusion Criteria:
- Confirmed diagnosis of chronic neuropathic pain
- Age 18–80 years
- Stable medication use for 30 days prior
- Ability to consent and comply with trial visits
Common Exclusion Criteria:
- Pacemakers or implanted stimulators
- Cognitive impairment
- Untreated psychological disorders
- Pregnancy or breastfeeding
Recruitment uses screening diagnostics, demographic quotas, and IRB-reviewed inclusion/exclusion frameworks.
Ethical Considerations in Clinical Trials for Pain Management
Clinical trial ethics safeguard both innovation and participant well-being.
Key Ethical Areas:
- Informed Consent: Ensures participants understand risks, benefits, and procedures
- IRB Oversight: Independent review of protocols to protect vulnerable populations
- Placebo Use Ethics: Balanced only when no proven treatment exists
- Clinical Equipoise: Only evaluate therapies whose efficacy is truly questionable.
Trials involving chronic pain patients must monitor psychological risk, adverse reactions, and maintain study integrity under ethical guidelines.
Clinical Trial Technologies and Innovations
Technology has transformed how Scrambler Therapy trials are conducted, monitored, and evaluated in 2025.
Technologies Powering Modern Trials:
- Wearables: Measure movement, sleep, and pain indicators
- ePRO (electronic patient-reported outcomes): Real-time symptom tracking
- Telemedicine: Remote check-ins, therapy delivery
- Digital Biomarkers: Identify treatment responsiveness
- EDC Systems: Centralized clinical data management
These innovations support decentralized trials, boosting participation and data quality.
Future Outlook of Clinical Trials in Pain Therapies
It appears that non-invasive therapies like scrambler therapy have a bright future.
What to Expect Post-2025:
- Expanded Indications: Investigations into CRPS, fibromyalgia, and phantom limb pain
- Next-Gen Devices: AI-driven signal modulation and adaptive algorithms
- Global Market Authorization: Pending EMA and FDA expansion pathways
- Increased Research Funding: Public and private research grants targeting pain management gaps
Forecast 2026 Pipeline includes 20+ trials exploring Scrambler Therapy as a first-line intervention in opioid-alternative pain protocols.
How to Find and Participate in Clinical Trials
Scrambler Therapy trial access is becoming more convenient through advocacy organizations and reliable venues.
How to Join a 2025 Clinical Trial:
- Use ClinicalTrials.gov: Search by condition, location, or keyword (e.g., “Scrambler Therapy pain”)
- Check NCT Numbers: Each trial has a unique identifier for tracking
- Reach Out to Trial Sites: Contact site coordinators or investigators
- Pre-Screen Online: Use digital eligibility checkers
- Speak With Your Doctor: Discuss trial fit and risks
Most studies offer participant compensation, care coverage, and access to new therapies.
Clinical Trials – FAQs (2025 Edition)
Is Scrambler Therapy FDA-approved or still experimental?
It is investigational for most conditions but has FDA Breakthrough Device Designation for some pain types.
What are the risks of joining a clinical trial for pain?
Risks are low; common side effects include minor skin irritation. All trials undergo IRB and safety board review.
How do I know if I qualify?
Check inclusion criteria on ClinicalTrials.gov, or contact a research site for pre-screening.
Does it cost to participate in a trial?
No. Most trials cover all costs and often offer travel reimbursement or compensation.
How long do Scrambler Therapy trials last?
Typically 4–12 weeks, including a treatment and follow-up period.
Will I get the results of the trial?
Participants usually receive summaries post-trial. Full data is often published in peer-reviewed journals or the ClinicalTrials.gov results section.
Experiencing Chronic Pain in South Florida?

Discover South Florida Scrambler Therapy is one of the nation’s leading clinics for noninvasive chronic pain relief, offering FDA-cleared Scrambler Therapy® for adults and children. Co-founded by Dr. Rick Markson, one of the few practitioners worldwide to receive advanced certification directly from the therapy’s inventor in Rome, our clinic delivers globally recognized expertise with compassionate, personalized care. If you or a loved one is living with treatment-resistant nerve pain, we invite you to schedule a consultation and explore a life beyond pain.
Recommended Reads:
📘 What is scrambler therapy?
📘 What to Expect During a Scrambler Session
📘 CRPS Pain Relief Without Drugs—Real Patient Stories
📘 Conditions that scrambler therapy can treat
Take the Next Step: Free Consultation at South Florida Scrambler

Every day counts when we suffer from chronic pain. South Florida Scrambler Therapy offers a free initial consultation to determine if Scrambler is right for you. Schedule Today:
- Speak directly with Dr. Rick Markson’s team
- Learn about treatment protocols and insurance
- Complete a customized treatment plan
- Start seeing results within days, not months
📞 Call Now or Visit website: www.southfloridascramblertherapy.com
📍 We serve Palm Beach, Fort Lauderdale, and Miami from our location at 100 NW 100th Ave, Plantation
You Can Follow Us through Our Social Media:
📸Instagram—Day-in-the-life stories from our patients
👍Facebook—Success journeys and community support
You deserve to laugh, and enjoy life without pain. The journey starts here.
Patient Reviews
Start Your Nerve Pain-Free Journey Today