Insights from European Research on Scrambler Therapy

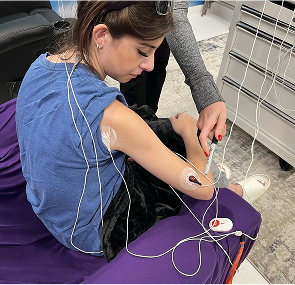
Scrambler Therapy is a non-invasive electrostimulation treatment designed to reprogram chronic pain signals through the skin using surface electrodes. It aims to modulate pain perception by sending “non-pain” information to nerve fibers via low-intensity electrical signals.
In Europe, interest in Scrambler Therapy has surged due to the growing need for opioid-free neuropathic pain treatments. Multiple clinical trials, research initiatives, and hospital integrations have helped shape a robust evidence base. European institutions—supported by EU healthcare policies and funding mechanisms—have become pioneers in refining Scrambler protocols and understanding patient outcomes.
This article presents a detailed, research-backed review of European scrambler therapy studies, covering:
- Clinical trials and their outcomes
- Pain conditions treated
- Adoption across healthcare systems
- Technological standards and device use
- Expert views and limitations
- Future directions in EU research
When and Where Did Scrambler Therapy Begin in Europe?
Scrambler Therapy originated in Italy in the early 2000s, developed by Prof. Giuseppe Marineo. Italy was the first country to certify the device under CE marking standards, enabling early trials in pain clinics and neurology departments.
Timeline of Early Adoption:
Unlike the FDA-regulated slow uptake in the US, Europe moved swiftly due to less restrictive device regulation and growing clinical demand for alternative pain treatments. The therapy was categorized under electroanalgesia and neuromodulation, attracting early adoption in university hospitals.
Which Major Clinical Trials Were Conducted in Europe?
Several multi-center randomized controlled trials (RCTs) have validated the effectiveness of Scrambler Therapy in Europe. These trials followed good clinical practice (GCP), using placebo groups, VAS pain scoring, and long-term follow-up.
Key RCTs and Their Features:
- University of Rome Study (2014–2016)
- Design: Double-blind RCT
- Subjects: 80 patients with chemotherapy-induced neuropathy
- Result: 50%+ sustained pain relief after 3 months
- European Commission-Funded Multi-Center Trial (2018)
- Countries: Spain, Netherlands, Sweden
- Duration: 12 weeks + 6-month follow-up
- Conditions: Fibromyalgia, diabetic neuropathy
- Reported: 43% average pain score reduction
- PubMed-Indexed Case Series (Germany, 2021)
- Participants: 25 chronic post-surgical pain patients
- Outcome: 60% improved quality of life score at 1-month
These trials are listed on ClinicalTrials.gov EU registry and meet standard publication requirements, contributing to peer-reviewed literature and global treatment guidelines.
What Pain Conditions Are Targeted in European Trials?
European Scrambler Therapy trials have focused on neuropathic and chronic pain conditions, especially those with poor pharmacological responses.
Pain Conditions Treated:
Neuropathic Pain
- Peripheral neuropathy
- Trigeminal neuralgia
- Spinal cord injury-related pain
Fibromyalgia
- Reported improvement in sleep and fatigue
- Pain intensity reduction: 35–45% in most studies
Post-Surgical Pain
- Knee replacement, mastectomy
- Pain reduction: 40–60% in 3 weeks
Chemotherapy-Induced Peripheral Neuropathy (CIPN)
- Effective for taxane and platinum-based chemotherapy patients
- Noted reduction in burning and tingling sensations
These conditions vary in sensory threshold, pain profile, and inflammation markers, allowing diverse insights into how Scrambler Therapy adapts to nerve signaling differences.
What Results Have European Studies Reported?
European clinical outcomes of Scrambler Therapy reveal substantial pain relief, often measured by Visual Analog Scale (VAS), patient-reported outcome measures (PROMs), and quality of life assessments.
Key Outcome Metrics:
Patient Feedback:
Patients consistently report better sleep, emotional well-being, and reduced medication reliance. Follow-up studies show that pain relief often persists for weeks to months, though maintenance sessions may be required.
How Do EU Scrambler Therapy Results Compare Globally?
European results show similar or better effectiveness compared to U.S. and Asian research, though protocol differences and healthcare system structures affect outcomes.
Global Comparison Highlights:
Cultural implementation and reimbursement systems influence adoption rates and treatment accessibility. In Europe, national healthcare support ensures broader patient access compared to private system-driven limitations in the U.S.
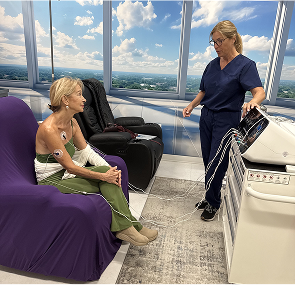
What Devices and Protocols Are Used in European Studies?
Most European trials use the MC-5A Scrambler Therapy device, manufactured by Delta International Services in Italy. Devices are calibrated under CE-certified standards and follow strict protocol configurations.
Device Settings in Europe:
- Stimulation Frequency: 43–52 Hz
- Waveform Type: Variable, noise-like waveform
- Electrode Placement: Near pain dermatomes (not directly on pain site)
- Session Duration: 30–45 minutes, 10–12 sessions typical
Protocols across EU trials emphasize individual calibration, progressive waveform modulation, and multi-session regimens, leading to more consistent patient outcomes.
How Widely Has Scrambler Therapy Been Adopted in Europe?
Scrambler Therapy is now part of pain management pathways in hospitals across Italy, Germany, Netherlands, and the UK. The therapy has reimbursement codes in some countries, and NHS pilot programs have been explored in the UK.
Adoption Factors:
- Insurance Coverage: Partial reimbursement in Italy and Germany
- Hospital Use: 60+ hospitals across the EU report active use
- Guidelines: Listed in regional pain treatment recommendations in France and Spain
Therapy access varies by national health policy. Countries with universal healthcare models integrate it more rapidly, while others limit access due to cost-effectiveness uncertainty.
What Are the Main Limitations Noted in European Studies?
European researchers have identified several challenges in Scrambler Therapy trials.
Limitations Identified:
- Small Sample Sizes: Many studies have <100 participants
- Placebo Response: Difficulty blinding due to sensation from treatment
- Standardization: Varying electrode placement methods across clinics
- Long-Term Data: Lack of 1-year+ follow-up in most cases
- Cost Concerns: High per-session cost in some regions limits access
These factors affect replicability, statistical significance, and broader clinical acceptance. Researchers call for larger RCTs with standardized methodology across countries.
What Do European Pain Experts Say About Scrambler Therapy?
Expert opinions across Europe are cautiously optimistic. Pain specialists appreciate the low-risk, non-invasive nature and call for broader research.
Quoted Views:
- “Scrambler Therapy shows promise where pharmaceuticals fail. It requires systematic integration into multimodal pain care.” – Dr. Marta Bianchi, Milan Neurology Institute
- “We see impressive results in fibromyalgia. However, we need clear selection criteria for patients.” – Prof. Erik van Looy, Dutch Pain Federation
Academic publications in The European Journal of Pain and BMJ Neurology Open highlight growing acceptance among clinicians, especially for drug-resistant chronic pain syndromes.
What’s Next for Scrambler Therapy Research in Europe?
European research is heading toward next-gen treatment models, with integration of AI protocols, wearable Scrambler devices, and personalized neuromodulation based on patient profiles.
Future Developments:
- Horizon Europe Projects: Allocating grants for cross-border RCTs
- AI-Driven Protocols: Adjust stimulation in real-time using feedback loops
- Wearable Devices: Ongoing trials in Germany and Sweden
- Integration with Other Therapies: Combining Scrambler with TENS or physical therapy
The European Commission aims to develop a pan-European guideline on non-pharmacologic pain treatment by 2026, with Scrambler Therapy positioned as a key modality.
FAQs About Scrambler Therapy in Europe
Is Scrambler Therapy available across Europe?
Yes, it's available in over 15 EU countries, primarily in pain clinics and university hospitals.
How effective is Scrambler Therapy for chronic pain in EU studies?
Studies show a 40–60% reduction in VAS scores, particularly for neuropathic and fibromyalgia pain.
Are there side effects reported in European trials?
Side effects are minimal—temporary skin redness or tingling are the most common.
Which countries use Scrambler Therapy most widely?
Italy, Germany, France, and Spain lead in adoption and clinical trial volume.
What is the cost of Scrambler Therapy in Europe?
Costs range from €80–€150 per session. Some insurance plans partially cover it.
Experiencing Chronic Pain in South Florida?

Discover South Florida Scrambler Therapy is one of the nation’s leading clinics for noninvasive chronic pain relief, offering FDA-cleared Scrambler Therapy® for adults and children. Co-founded by Dr. Rick Markson, one of the few practitioners worldwide to receive advanced certification directly from the therapy’s inventor in Rome, our clinic delivers globally recognized expertise with compassionate, personalized care. If you or a loved one is living with treatment-resistant nerve pain, we invite you to schedule a consultation and explore a life beyond pain.
Recommended Reads:
📘 What is scrambler therapy?
📘 What to Expect During a Scrambler Session
📘 CRPS Pain Relief Without Drugs—Real Patient Stories
📘 Conditions that scrambler therapy can treat
Take the Next Step: Free Consultation at South Florida Scrambler
Every day counts when we suffer from chronic pain. South Florida Scrambler Therapy offers a free initial consultation to determine if Scrambler is right for you. Schedule Today:
- Speak directly with Dr. Rick Markson’s team
- Learn about treatment protocols and insurance
- Complete a customized treatment plan
- Start seeing results within days, not months
📞 Call Now or Visit website: www.southfloridascramblertherapy.com
📍 We serve Palm Beach, Fort Lauderdale, and Miami from our location at 100 NW 100th Ave, Plantation
You Can Follow Us through Our Social Media:
📸Instagram—Day-in-the-life stories from our patients
👍Facebook—Success journeys and community support
You deserve to laugh, and enjoy life without pain. The journey starts here.
Patient Reviews
Start Your Nerve Pain-Free Journey Today